Basal ganglia
| Brain: Basal ganglia | ||
|---|---|---|
 |
||
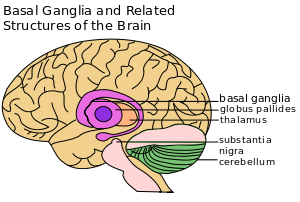
| Basal ganglia labeled at top right. | ||
| Latin | nuclei basales | |
| NeuroNames | hier-206 | |
| MeSH | Basal+Ganglia | |
| NeuroLex ID | birnlex_826 | |
The basal ganglia (or basal nuclei) are a group of nuclei in the brains of vertebrates. They are situated at the base of the forebrain and strongly connected with the cerebral cortex, thalamus and other areas. The basal ganglia are associated with a variety of functions, including motor control and learning. Currently popular theories implicate the basal ganglia primarily in action selection, that is, the decision of which of several possible behaviors to execute at a given time. Experimental studies show that the basal ganglia exert an inhibitory influence on a number of motor systems, and that a release of this inhibition permits a motor system to become active. The "behavior switching" that takes place within the basal ganglia is influenced by signals from many parts of the brain, including the prefrontal cortex, which is widely believed to play a key role in executive functions.
The main components of the basal ganglia are the striatum, pallidum, substantia nigra, and subthalamic nucleus. The largest component, the striatum, receives input from many brain areas but sends output only to other components of the basal ganglia. The pallidum receives its most important input from the striatum (either directly or indirectly), and sends inhibitory output to a number of motor-related areas, including the part of the thalamus that projects to the motor-related areas of the cortex. The substantia nigra consists of two parts, one that functions similarly to the pallidum, and another that provides the source of dopamine input to the striatum. The subthalamic nucleus receives input mainly from the striatum and cortex, and projects to the pallidum. Each of these areas has a complex internal anatomical and neurochemical organization.
The basal ganglia play a central role in a number of neurological conditions, including several movement disorders. The most notable are, first, Parkinson's disease, involving degeneration of the melanin-pigmented dopamine-producing cells in the substantia nigra, and secondly, Huntington's disease, which primarily involves damage to the striatum. Basal ganglia dysfunction is also implicated in some other disorders of behavior control such as Tourette's syndrome and obsessive–compulsive disorder, although the neural mechanisms underlying these are not well understood.
The basal ganglia have a limbic sector whose components are assigned distinct names: the nucleus accumbens (NA), ventral pallidum, and ventral tegmental area (VTA). VTA efferents provide dopamine to the nucleus accumbens (ventral striatum) in the same way that the substantia nigra provides dopamine to the dorsal striatum. Because there is much evidence that it plays a central role in reward learning, the VTA→NA dopaminergic projection has attracted a great deal of attention. For example, a number of highly addictive drugs, including cocaine, amphetamines, and nicotine, are thought to work by increasing the efficacy of the VTA→NA dopamine signal. There is also evidence implicating overactivity of the VTA dopaminergic projection in schizophrenia.
Contents |
Anatomy
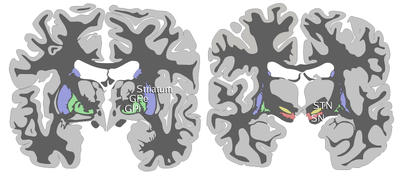
ANTERIOR: striatum, globus pallidus (GPe and GPi)
POSTERIOR: subthalamic nucleus (STN), substantia nigra (SN)
The basal ganglia form a fundamental component of the vertebrate telencephalon (forebrain). In contrast to the pallial or cortical layer that lines the surface of the forebrain, the basal ganglia are a collection of distinct masses of gray matter lying in the interior, not far from the junction with the thalamus. Like most parts of the brain, the basal ganglia consist of left and right sides that are virtual mirror images of each other.
At the highest level, the basal ganglia are divided by anatomists into four distinct structures. Two of them, the striatum and pallidum, are relatively large; the other two, the substantia nigra and subthalamic nucleus, are smaller. In the illustration to the right, two coronal sections of the human brain show the location of the basal ganglia. The subthalamic nucleus and substantia nigra lie farther back in the brain than the striatum and pallidum.
Connections

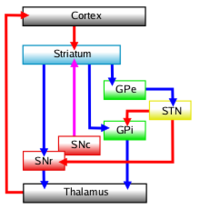
The flow of neural signals through the basal ganglia is strongly directional. The striatum is the primary recipient of input from other brain areas, most notably the cerebral cortex. The internal segment of the globus pallidus (GPi), together with the reticular part of the substantia nigra (SNr), give rise to the primary output, most notably to the thalamus. The striatum projects to the pallidum both directly and indirectly via the subthalamic nucleus, which also receives cortical input. The substantia nigra consists of two parts, one of which functions similarly to the pallidum, the other of which sends a modulatory dopaminergic input to the striatum and other structures.
The adjoining figures show some of the most important connections between components. On the largest scale, the basal ganglia form a loop that begins and ends in the cortex. Anatomists have distinguished two main circuits, known as the "direct" and "indirect" pathways. The direct pathway runs cortex→striatum→GPi→thalamus→cortex. Two of these links are excitatory, and two inhibitory, so the net effect of the whole sequence is excitatory: the cortex excites itself via the direct pathway. The indirect pathway runs cortex→striatum→GPe→STN→GPi→thalamus→cortex. Three of these links are inhibitory and two excitatory, so the net effect of the sequence is inhibitory: the cortex inhibits itself via the indirect pathway. The total effect of basal ganglia upon the cortex is believed to result from a complex interplay between these two pathways.
Striatum
The striatum is the largest component of the basal ganglia. The term "striatum" comes from the observation that this structure has a striped appearance when sliced in certain directions, arising from numerous large and small bundles of nerve fibers (white matter) that traverse it. Early anatomists, examining the human brain, perceived the striatum as two distinct masses of gray matter separated by a large tract of white matter called the internal capsule. They named these two masses the "caudate nucleus" and "putamen". More recent anatomists have concluded, on the basis of microscopic and neurochemical studies, that it is more appropriate to consider these masses as two separated parts of a single entity, the "striatum", in the same way that a city may be separated into two parts by a river. Numerous functional differences between the caudate and putamen have been identified, but these are taken to be consequences of the fact that each sector of the striatum is preferentially connected to specific parts of the cerebral cortex.
The internal organization of the striatum is extraordinarily complex. The great majority of neurons (about 96%) are of a type called "medium spiny neurons". These are GABAergic cells (meaning that they inhibit their targets) with small cell bodies and dendrites densely covered with dendritic spines, which receive synaptic input primarily from the cortex and thalamus. Medium spiny neurons can be divided into subtypes in a number of ways, on the basis of neurochemistry and connectivity. The next most numerous type (around 2%) are a class of large cholinergic interneurons with smooth dendrites. There are also several other types of interneurons making up smaller fractions of the neural population.
Numerous studies have shown that the connections between cortex and striatum are generally topographic; that is, each part of the cortex sends stronger input to some parts of the striatum than to others. The nature of the topography has been difficult to understand, however—perhaps in part because the striatum is organized in three dimensions whereas the cortex, as a layered structure, is organized in two. This dimensional discrepancy entails a great deal of distortion and discontinuity in mapping one structure to the other.
Pallidum
The pallidum consists of a large structure called the globus pallidus ("pale globe") together with a smaller ventral extension called the ventral pallidum. The globus pallidus appears as a single neural mass, but can be divided into two functionally distinct parts, called the internal (sometimes "medial") and external (sometimes "lateral") segments, abbreviated GPi and GPe. Both segments contain primarily GABAergic neurons, which therefore have inhibitory effects on their targets. The two segments participate in distinct neural circuits. The external segment, or GPe, receives input mainly from the striatum, and projects to the subthalamic nucleus. The internal segment, or GPi, receives signals from the striatum via two pathways, called "direct" and "indirect". The direct pathway consists of direct projections from the striatum to the GPi. The indirect pathway consists of projections from the striatum to the GPe, followed by projections from the GPe to the subthalamic nucleus (STN), followed by projections from the STN to the GPi. These pathways have opposite net effects: striatal activity inhibits the GPi via the direct pathway because striatal outputs are GABAergic, but has a net excitatory effect on the GPi via the indirect pathway because this three-link pathway consists of two inhibitory links plus one excitatory link.
Pallidal neurons operate using a "disinhibition" principle. These neurons fire at steady high rates in the absence of input, and signals from the striatum cause them to "pause". Because pallidal neurons themselves have inhibitory effects on their targets, the net effect of striatal input to the pallidum is a reduction of the tonic inhibition exerted by pallidal cells on their targets.
Substantia nigra
Subthalamic nucleus
A note concerning terminology
The nomenclature of the basal ganglia system and its components has always been problematic. Early anatomists, seeing the macroscopic anatomical structure but knowing nothing of the cellular architecture or neurochemistry, grouped together components that are now believed to have distinct functions (such as the internal and external segments of the globus pallidus), and gave distinct names to components that are now thought to be functionally parts of a single structure (such as the caudate nucleus and putamen).
The term "basal" comes from the fact that most of its elements are located in the basal part of the forebrain. The term ganglia is a misnomer: in modern usage, neural clusters are only called "ganglia" in the peripheral nervous system; in the central nervous system they are called "nuclei". For this reason, the basal ganglia are also occasionally known as the "basal nuclei".[1] Terminologia anatomica (1998), the international authority for anatomical naming, retained "nuclei basales", but this is not commonly used.
The International Basal Ganglia Society (IBAGS) informally considers the basal ganglia to be made up of the striatum, the pallidum (with two nuclei), the substantia nigra (with its two distinct parts) and the subthalamic nucleus. Percheron et al. in 1991 and Parent and Parent in 2005 included the central region (centre median-parafascicular) of the thalamus as part of the basal ganglia[2][3], while Mena-Segovia et al. in 2004 included the pedunculopontine complex as well.[4]
Also, the names given to the various nuclei of the basal ganglia are different in different species. In particular, the internal segment of the globus pallidus in primates is called the entopeduncular nucleus in rodents. The "striatum" and "external segment of the globus pallidus" in primates are called the "paleostriatum augmentatum" and "paleostriatum primitivum" respectively in birds.
Function
Information about the functions of the basal ganglia comes from anatomical studies, from physiological studies carried out mainly in rats and monkeys, and from the study of diseases that damage them.
The greatest source of insight into the functions of the basal ganglia has come from the study of two neurological disorders, Parkinson's disease and Huntington's disease. For both of these disorders, the nature of the neural damage is well understood and can be correlated with the resulting symptoms. Parkinson's disease involves major loss of dopaminergic cells in the substantia nigra; Huntington's disease involves massive loss of medium spiny neurons in the striatum. The symptoms of the two diseases are virtually opposite: Parkinson's disease is characterized by gradual loss of the ability to initiate movement, while Huntington's disease is characterized by an inability to prevent parts of the body from moving unintentionally. It is noteworthy that although both diseases have cognitive symptoms, especially in their advanced stages, the most salient symptoms relate to the ability to initiate and control movement. Thus, both are classified primarily as movement disorders. A different movement disorder, called hemiballismus, may result from damage restricted to the subthalamic nucleus. Hemiballismus is characterized by violent and uncontrollable flinging movements of the arms and legs.
Eye movements
One of the most intensively studied functions of the BG is their role in controlling eye movements.[5] Eye movement is influenced by an extensive network of brain regions that converge on a midbrain area called the superior colliculus (SC). The SC is a layered structure whose layers form two-dimensional retinotopic maps of visual space. A "bump" of neural activity in the deep layers of the SC drives an eye movement directed toward the corresponding point in space.
The SC receives a strong inhibitory projection from the BG, originating in the substantia nigra pars reticulata (SNr).[5] Neurons in the SNr usually fire continuously at high rates, but at the onset of an eye movement they "pause", thereby releasing the SC from inhibition. Eye movements of all types are associated with "pausing" in the SNr; however, individual SNr neurons may be more strongly associated with some types of movements than others. Neurons in some parts of the caudate nucleus also show activity related to eye movements. Since the great majority of caudate cells fire at very low rates, this activity almost always shows up as an increase in firing rate. Thus, eye movements begin with activation in the caudate nucleus, which inhibits the SNr via the direct GABAergic projections, which in turn disinhibits the SC.
Role in motivation
Although the role of the basal ganglia in motor control is clear, there are also many indications that it is involved in the control of behavior in a more fundamental way, at the level of motivation. In Parkinson's disease, the ability to execute the components of movement is not greatly affected, but motivational factors such as hunger fail to cause movements to be initiated or switched at the proper times. The immobility of Parkinsonian patients has sometimes been described as a "paralysis of the will".[6] These patients have occasionally been observed to show a phenomenon called kinesia paradoxica, in which a person who is otherwise immobile responds to an emergency in a coordinated and energetic way, then lapses back into immobility once the emergency has passed.
The role in motivation of the "limbic" part of the basal ganglia—the nucleus accumbens (NA), ventral pallidum, and ventral tegmental area (VTA)—is particularly well established. Thousands of experimental studies combine to demonstrate that the dopaminergic projection from the VTA to the NA plays a central role in the brain's reward system. Animals with stimulating electrodes implanted along this pathway will bar-press very energetically if each press is followed by a brief pulse of electrical current. Numerous things that people find rewarding, including addictive drugs, good-tasting food, and sex, have been shown to elicit activation of the VTA dopamine system. Damage to the NA or VTA can produce a state of profound torpor.
Although it is not universally accepted, some theorists have proposed a distinction between "appetitive" behaviors, which are initiated by the basal ganglia, and "consummatory" behaviors, which are not. For example, an animal with severe basal ganglia damage will not move toward food even if it is placed a few inches away, but if the food is placed directly in the mouth, the animal will chew it and swallow it.
Comparative anatomy and naming
The basal ganglia form one of the basic components of the forebrain, and can be recognized in all species of vertebrates.[7] Even in the lamprey (generally considered one of the most primitive of vertebrates), striatal, pallidal, and nigral elements can be identified on the basis of anatomy and histochemistry.[8]
A clear emergent issue in comparative anatomy of the basal ganglia is the development of this system through phylogeny as a convergent cortically re-entrant loop in conjunction with the development and expansion of the cortical mantle. There is controversy, however, regarding the extent to which convergent selective processing occurs versus segregated parallel processing within re-entrant closed loops of the basal ganglia. Regardless, the transformation of the basal ganglia into a cortically re-entrant system in mammalian evolution occurs through a re-direction of pallidal (or "paleostriatum primitivum") output from midbrain targets such as the superior colliculus, as occurs in sauropsid brain, to specific regions of the ventral thalamus and from there back to specified regions of the cerebral cortex that form a subset of those cortical regions projecting into the striatum. The abrupt rostral re-direction of the pathway from the internal segment of the globus pallidus into the ventral thalamus—via the path of the ansa lenticularis--could be viewed as a footprint of this evolutionary transformation of basal ganglia outflow and targeted influence. The evolutionary emergence of cortical re-entrant systems in the brain has been postulated by Gerald Edelman as a critical basis for the emergence of primary consciousness in the theory of Neural Darwinism.
Neurotransmitters
In most regions of the brain, the predominant classes of neurons use glutamate as neurotransmitter and have excitatory effects on their targets. In the basal ganglia, however, the great majority of neurons use GABA as neurotransmitter and have inhibitory effects on their targets. The inputs from the cortex and thalamus to the striatum and STN are glutamatergic, but the outputs from the striatum, pallidum, and substantia nigra pars reticulata all use GABA. Thus, following the initial excitation of the striatum, the internal dynamics of the basal ganglia are dominated by inhibition and disinhibition.
Other neurotransmitters have important modulatory effects. The most intensively studied is dopamine, which is used by the projection from the substantia nigra pars compacta to the striatum, and also in the analogous projection from the ventral tegmental area to the nucleus accumbens. Acetylcholine also plays an important role, being used both by several external inputs to the striatum, and by a group of striatal interneurons. Although cholinergic cells make up only a small fraction of the total population, the striatum has one of the highest acetylcholine concentrations of any brain structure.
Disorders associated with the basal ganglia
- Attention-deficit hyperactivity disorder (ADHD)
- Athymhormic syndrome (PAP syndrome)
- Cerebral palsy: basal ganglia damage during second and third trimester of pregnancy
- Dystonia
- Fahr's disease
- Foreign accent syndrome (FAS)
- Huntington's disease
- Lesch-Nyhan syndrome
- Obsessive-compulsive disorder[9][10]
- Other anxiety disorders [10]
- Parkinson's disease
- PANDAS
- Sydenham's chorea
- Tourette's disorder
- Tardive dyskinesia, caused by chronic antipsychotic treatment
- Stuttering[11]
- Spasmodic dysphonia
- Wilson's disease
- Blepharospasm
History
The acceptance that the basal ganglia system constitutes one major cerebral system took long to arise. The first anatomical identification of distinct subcortical structures was published by Thomas Willis in 1664.[12] For many years, the term corpus striatum[13] was used to describe a large group of subcortical elements, some of which were later discovered to be functionally unrelated.[14] For many years, the putamen and the caudate nucleus were not associated with each other. Instead, the putamen was associated with the pallidum in what was called the nucleus lenticularis or nucleus lentiformis.
A thorough reconsideration by Cécile and Oskar Vogt (1941) simplified the description of the basal ganglia by proposing the term striatum to describe the group of structures consisting of the caudate nucleus, the putamen and the mass linking them ventrally, the nucleus accumbens. The striatum was named on the basis of the striated (striped) appearance created by radiating dense bundles of striato-pallido-nigral axons, described by anatomist Samuel Alexander Kinnier Wilson (1912) as "pencil-like".
The anatomical link of the striatum with its primary targets, the pallidum and the substantia nigra was discovered later. The name globus pallidus was attributed by Déjerine to Burdach (1822). For this, the Vogts proposed the simpler "pallidum". The term "locus niger" was introduced by Félix Vicq-d'Azyr as tache noire in (1786), though that structure has since become known as the substantia nigra, due to Von Sömmering in 1788. The structural similarity between the substantia nigra and globus pallidus was noted by Mirto in 1896. Together, the two are known as the pallidonigral ensemble, which represents the core of the basal ganglia. Altogether, the main structures of the basal ganglia are linked to each other by the striato-pallido-nigral bundle, which passes through the pallidum, crosses the internal capsule as the "comb bundle of Edinger", then finally reaches the substantia nigra.
Additional structures that later became associated with the basal ganglia are the "body of Luys" (1865) (nucleus of Luys on the figure) or subthalamic nucleus, whose lesion was known to produce movement disorders. More recently, other areas such as the central complex (centre médian-parafascicular) and the pedunculopontine complex have been thought to be regulators of the basal ganglia.
Near the beginning of the 20th century, the basal ganglia system was first associated with motor functions, as lesions of these areas would often result in disordered movement in humans (chorea, athetosis, Parkinson's disease).
See also
- Anatomical subdivisions and connections of the basal ganglia
- Nathaniel A. Buchwald
- Primate basal ganglia system
References
- ↑ Soltanzadeh, Akbar (2004). Neurologic Disorders. Tehran: Jafari. ISBN 964-6088-03-1.
- ↑ Percheron et al. (1991)
- ↑ Parent and Parent (2005)
- ↑ Mena-Segovia et al. (2004)
- ↑ 5.0 5.1 PMID 10893428 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 17978012 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Parent A (1986). Comparative Neurobiology of the Basal Ganglia. Wiley. ISBN 9780471803485.
- ↑ PMID 9651523 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Radua, Joaquim; Mataix-Cols, David (November 2009). "Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder". British Journal of Psychiatry 195 (5): 393–402. doi:10.1192/bjp.bp.108.055046. PMID 19880927.
- ↑ 10.0 10.1 Radua, Joaquim; van den Heuvel, Odile A.; Surguladze, Simon; Mataix-Cols, David (5 July 2010). "Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders". Archives of General Psychiatry 67 (7): 701–711. doi:10.1001/archgenpsychiatry.2010.70. PMID 20603451.
- ↑ Alm PA (2004). "Stuttering and the basal ganglia circuits: a critical review of possible relations". Journal of communication disorders 37 (4): 325–69. doi:10.1016/j.jcomdis.2004.03.001. PMID 15159193. http://theses.lub.lu.se/scripta-archive/2005/02/02/med_1035/part2/Per_Alm_Paper_II.pdf.
- ↑ Andrew Gilies, A brief history of the basal ganglia, retrieved on 27 June 2005
- ↑ Vieussens, 1685
- ↑ Percheron et al. (1994)
External links
- Basal+ganglia at eMedicine Dictionary
- Imaging of Basal Ganglia at USUHS
- Scholarpedia article on Basal ganglia
- NIF Search - Basal Ganglia via the Neuroscience Information Framework
- The International Basal Ganglia Society
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||